(Rogers, Hale and Wszelaki)
Chapter 1: Efficacy of a new bacterial biopesticide, MBI-203 and Beauveria bassiana for cucumber beetle management on pumpkins and melons.
Objectives:
• Examine the efficacy of an early stage biopesticide in managing two species of diabroticite beetles spotted and striped cucumber beetles (Diabrotica undecimpunctata and Acalymma vittata) on melon and pumpkin plants in the field
• Compare 3 different rates of this novel biopesticide with the label rate of Beauveria bassiana (Mycotrol-O) and Carbaryl (Sevin 80 S)
• Determine if beetle densities are impacted by alternating commonly used plant protectants with a novel biopesticide in an integrated pest management program for sustainable melon and pumpkin production
On May 18-20, 410 each of pumpkin (‘Baby Pam’) and melon (Galia ‘Diplomat’ F1) untreated seeds (Johnny’s Selected Seeds) were started in 4.5” pots in the greenhouse. SunGro Sunshine Organic Blend Professional Growing Mix (Sun Gro Horticultural, Bellevue, WA) was used as the potting media. Seedlings were hand fertilized on May 27, June 3 and June 9 with 200 mL Rain Grow 4-2-3 liquid fertilizer (Oliver, BC, Canada) in 1 L water delivering 75 mL per pot. The greenhouse temperature settings are 65° F/ 70° F night/day with a photoperiod of 16:8. The greenhouse temperature fluctuates 4-5 degrees cooler than outside air temperatures.
Plots were flagged out in a 140’ x 450’ non-certified organic plot of land at the UT Organic Crops Unit (range L). A cowpea cover crop was flail mowed (Alamo SH74, Alamo Industrial, Seguin, TX) and tilled on May 28 with a rotary tiller (Bush Hog, Selma, AL), and again on June 14. On June 14, 4’ wide plastic mulch and dripline irrigation were laid in rows. Pumpkin plots were 40’ long with 10’ in between rows. Melon plots were 20’ long with 10’ in between rows. A 4’ wide strip of buckwheat was seeded on either side of pumpkin/melon plots with an Almaco light duty grain drill (Almaco, Nevada, IA) at the rate of 40 lbs per acre. Pumpkin plants were set out in the field by hand on June 15, and melon plants on June 16. There were four replicates of each crop, with 10 plants in each plot. Pumpkin plants were set 4’ apart in-row, and melon plants were set 2’ apart in-row. At planting, 40 g of 10-4-4 Nature Safe course ground fertilizer (Griffin Industries Inc., Cold Spring, KY) was side dressed to each transplant by hand. Plants were side dressed when flowering on July 14 (3rd and 4th replicate of pumpkins received 80 g of Nature Safe 10-8-5, with 1st and 2nd replicates and melon plants receiving 100 g of soybean meal 7-2-1 (TN Farmers Co-op).
Spray treatments consisted of a novel biopesticide, MBI 203 EP, comprised of a Chromobacteria substugae (94.5% AI) developed by Marrone Bio Innovations (Davis, CA). The field rates of MBI 203 EP initially proposed were 1X (5% v/v), 2X (10% v/v) and 4X (20% v/v) but were changed to 1X and 3X (15% v/v) due to limited availability of the product.
Beauveria bassiana strain GHA (Mycotrol-O, AI 10.9%, Laverlam International, Butte, MT) was used in field rates of 1X (1 qt/acre) and 2X (2 qt/acre).
Carbaryl (Sevin concentrate, 22.5% AI, TechPac LLC, Lexington, KY) was used at the 1X label rate (1.5 fl oz per 1000 ft2).
The treatments were:
- Mycotrol-O label rate, B1X (17.39 mL of product in 1.6 L)
- Mycotrol-O double rate, B2X (34.79 mL of product in 1.6 L)
- unsprayed control, C
- MBI 203 label rate, M1X (80 mL of product in 1.6 L)
- MBI 203 triple rate, M3X (240 mL of product in 1.6 L)
- MBI 203 label rate alternated with Mycotrol-O label rate, MB
- MBI 203 label rate alternated with Sevin label rate, MSV
- Sevin label rate, SV (35.51 mL of product in 1.6 L)
The spray treatments began on July 2 and were repeated weekly for 6 weeks (July 2, July 7, July 14, July 22, July 28 and Aug 4). For the last three spray dates, the MBI 203 3X rate was reduced to 2X (10% v/v) due to limited supply of the product.
A backpack sprayer with 5 lb CO2 cylinder, 60 PSI regulator, and 4 nozzle boom (19” spacing) was used (Bellspray Inc., Opelousas, LA). Spray formulations were mixed in 2-L plastic soda bottles. The sprayer was calibrated to deliver 23 gal/acre at walking speed. Boom was held at 18” above plants.
Insect scouting for spotted (Diabrotica undecimpunctata) and striped (Acalymma vittata) beetles began on June 29. Two random plants (20% of plants) were visually inspected on pumpkins and melons. In addition to cucumber beetles, squash bugs (Anasa tristis) and squash vine borer (Melittia cucurbitae) were also counted. Insect scouting was repeated weekly for eight weeks (last scouting date was Aug 10).
Beneficial insects and pollinators were visually scouted in crops weekly and monitored in the buckwheat alleys using yellow sticky cards set out on July 23 (64 cards, one in each row) and Aug 3 (32 cards, every other buckwheat row). Cards were collected after 5 days and kept in the freezer for identification. Bees and beneficial insects were also monitored in the buckwheat alleys using visual observations on Aug 5 and Aug 12. A transect of 40’ for buckwheat alleys beside pumpkins rows and 20’ for alleys beside melon rows was used, with sampling occurring every 6’ and 3’ respectively. Beneficial insects (honeybees, bumblebees, sweat bees, other bees, flies and lady beetles) were counted for 15 seconds at each sampling point. Monitoring was done from 9:30-11:30 am.
Harvesting began for melons on July 23. Melons were harvested twice weekly until Aug 19, eight harvests total. Pumpkin harvesting began on June 29 and continued twice weekly until Aug 23, six harvests total. Total fruit per plot was sorted into marketable and unmarketable categories, counted and weighed.
1.1 Insect counts
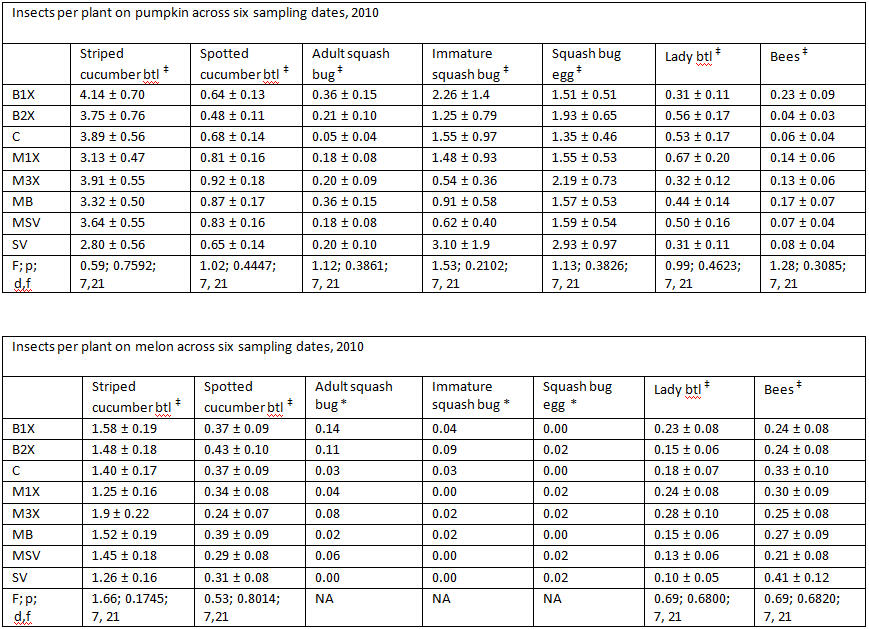
* values in these columns are raw means, analysis could not be done due to low populations
‡ analysis was done using PROC GLIMMIX (Generalized Linear Mixed Models) ANOVA in SAS (9.1, Cary, NC), α = 0.05.
1.2 Insect populations on pumpkin plants
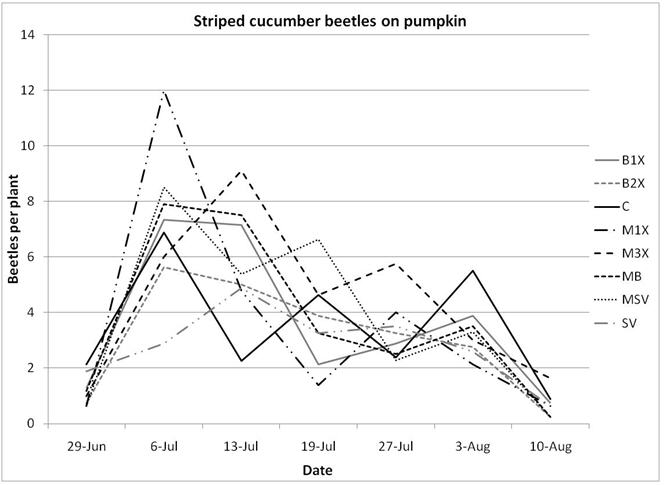
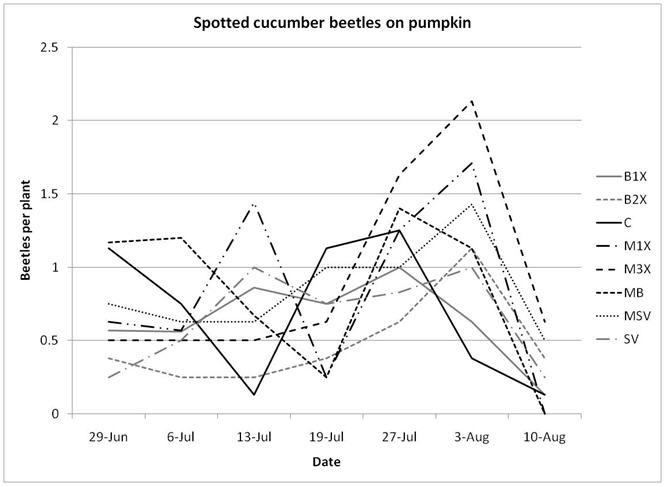
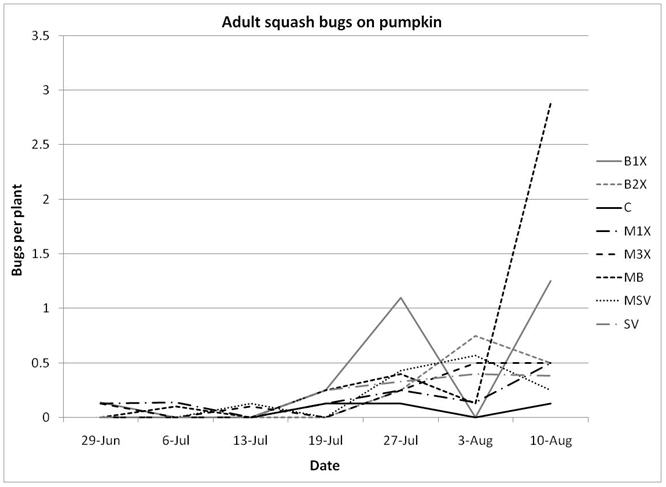
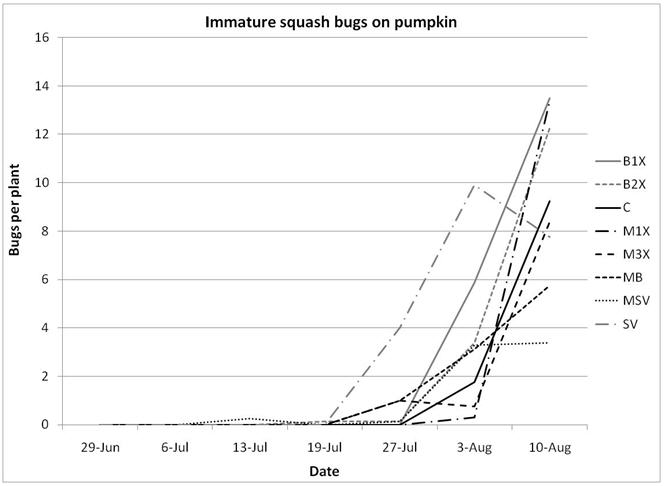
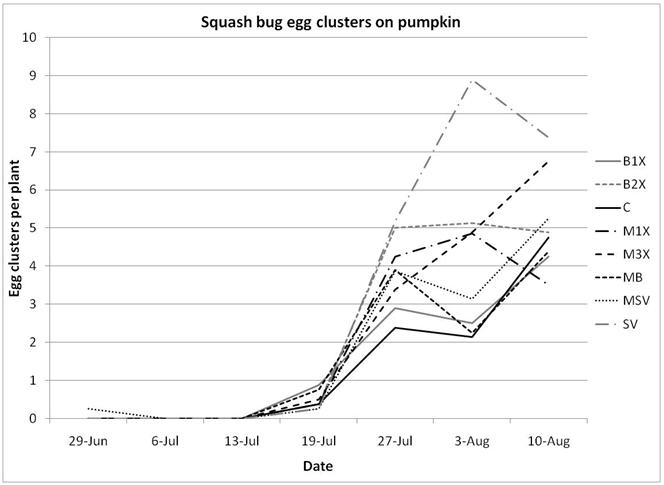
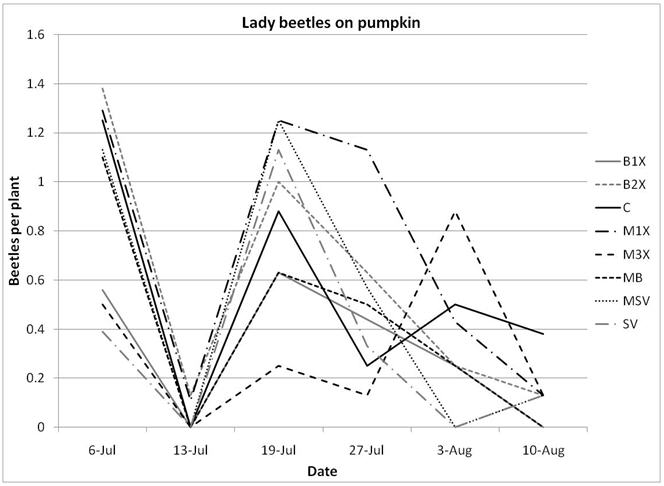
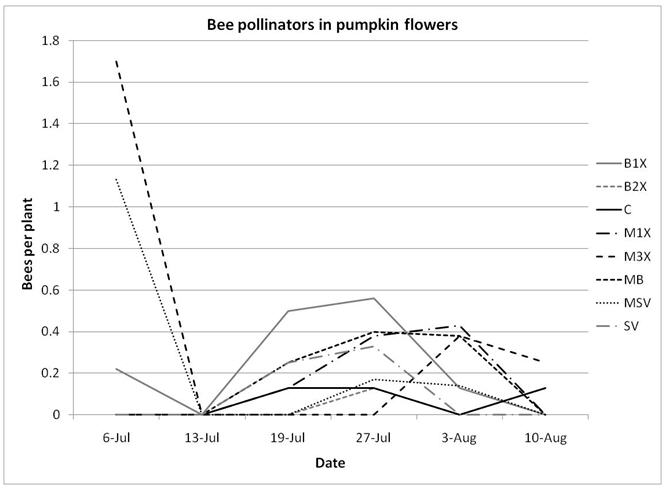
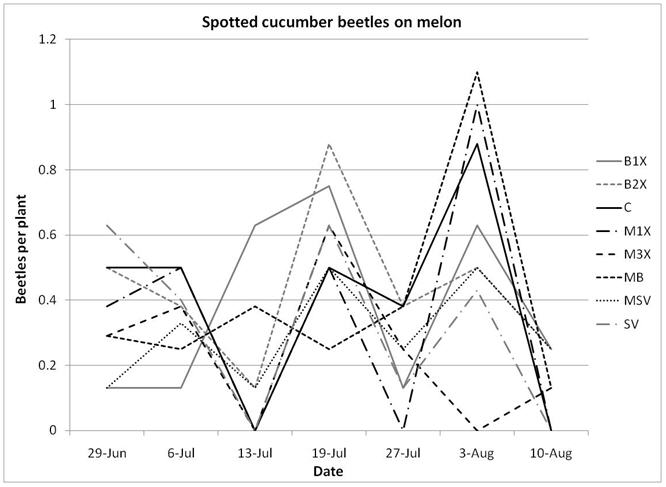
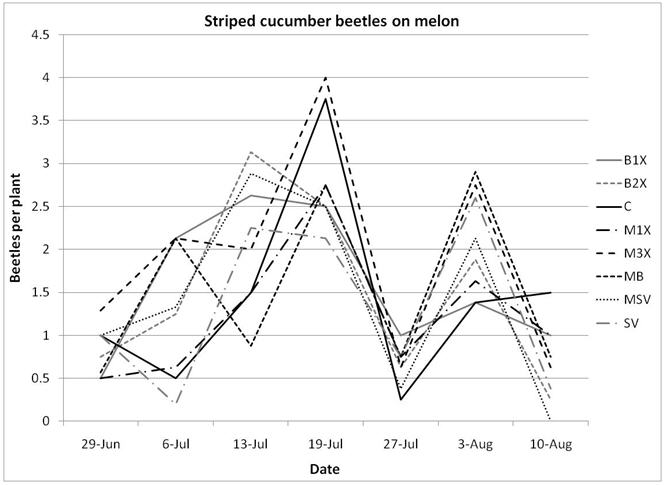
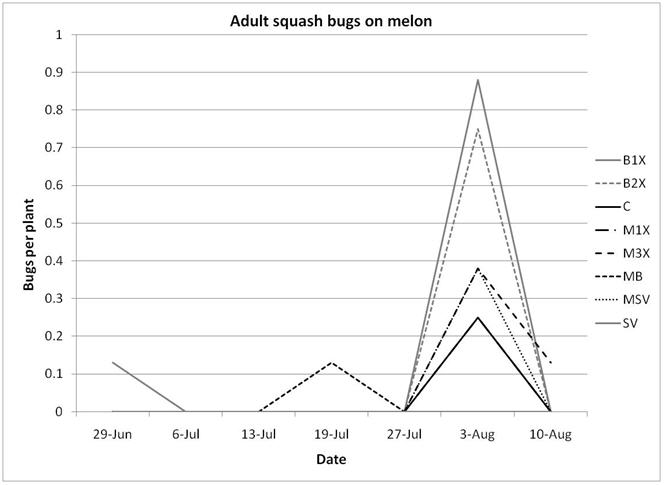
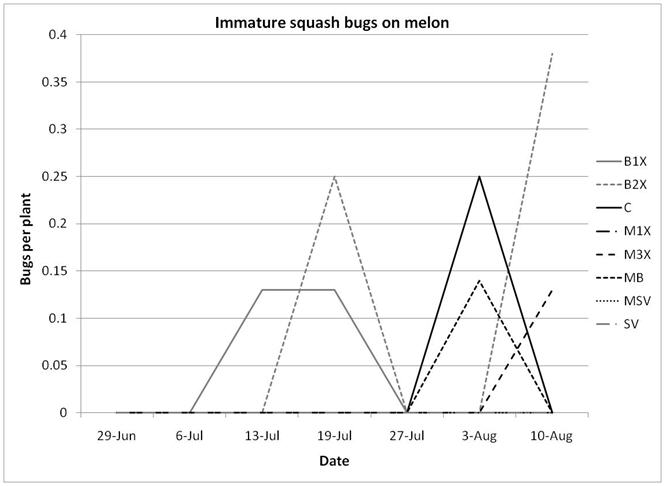
1.3 Insect populations on melon plants
1.4 Harvest data
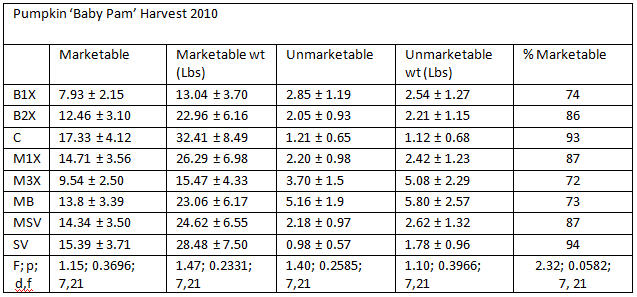
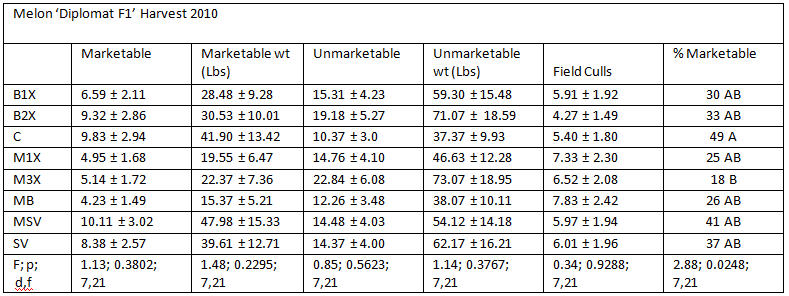
Analysis was done using PROC MIXED (Mixed Models) ANOVA in SAS (9.1, Cary, NC), α = 0.05. Values followed by different letters are significantly different using Tukey’s LSD.
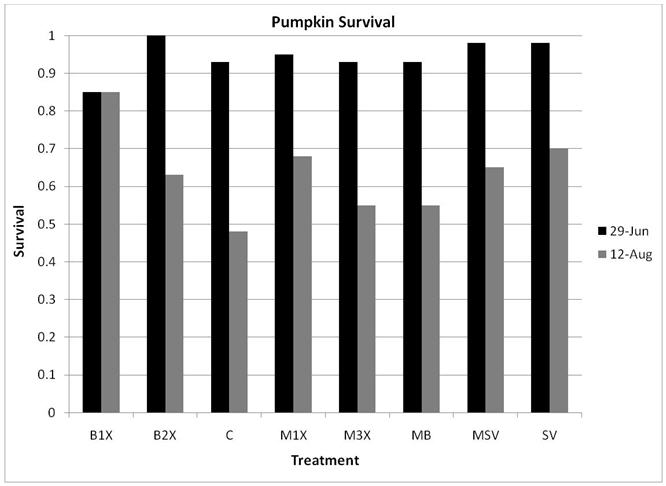
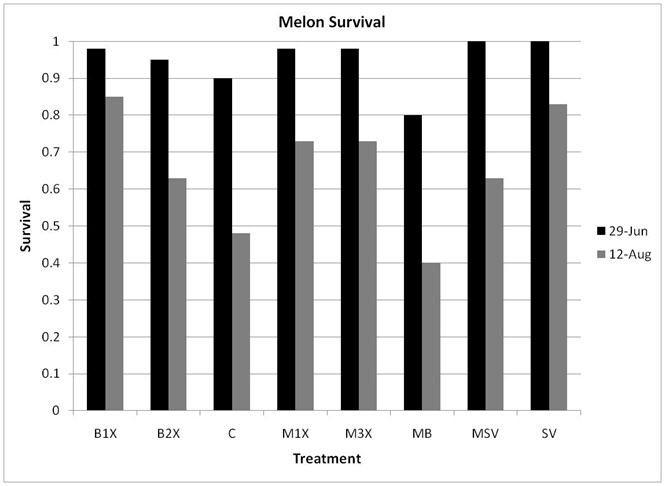
1.5 Plant Survival
1.1 Insect counts
There were noticeably more cucumber beetles on pumpkin plants than on melons. Of the cucumber beetles, the striped (Acalymma vittata) was detected more often than the spotted (Diabrotica undecimpunctata), especially on pumpkins, where the majority of beetles were found inside flowers. Although slightly fewer striped cucumber beetles were found in the SV (Sevin) treatments, there was no significant difference between the eight treatments, including the unsprayed control plots.
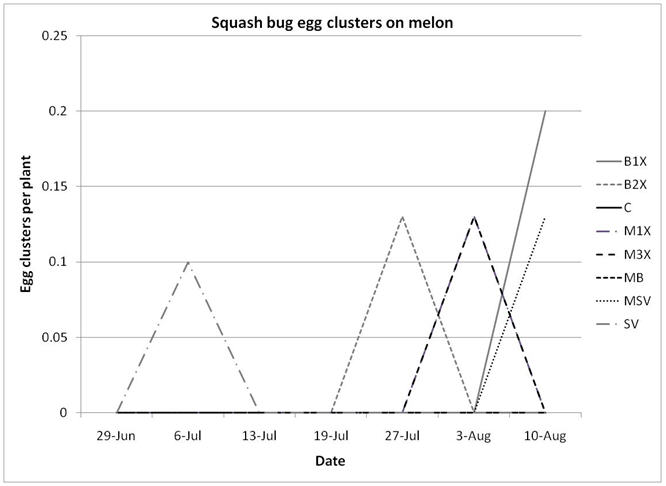
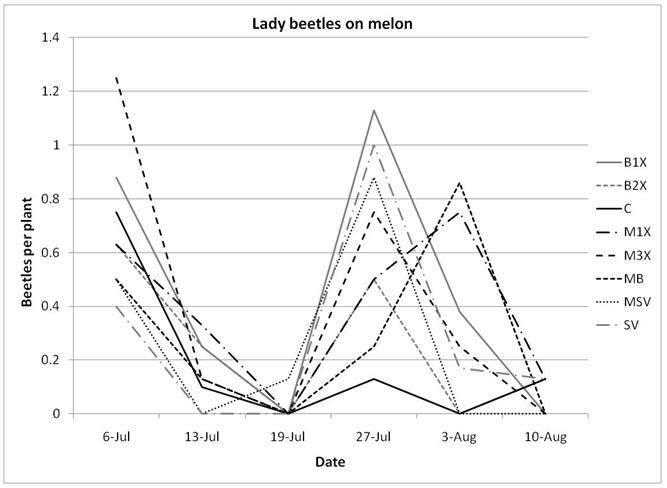
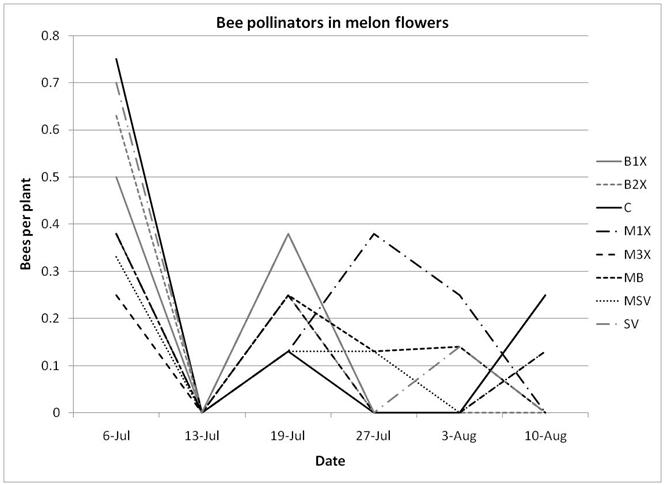
Squash bug (Anasa tristis) adults, juveniles and eggs were also denser in pumpkin plants than in melons, where they were found in very low numbers. This reflects host preferences, as squash bugs will feed on melons but are unable to complete their lifecycle on them. Immature squash bugs and squash bug eggs were slightly higher on SV treatments, although carbaryl targets these pests. There was no significant difference in squash bug populations between spray treatments. The eggs and juveniles are often found on the undersides of leaves, where they may be protected from direct contact with foliar sprays. There were no differences in lady beetles or bee pollinators between spray treatments for either pumpkin or melons.
1.2 Insect populations on pumpkin plants
Insect populations were highly variable across sampling dates, with striped beetle populations slightly decreasing, spotted beetles roughly staying unchanged, and squash bugs increasing over time. Very few squash vine borers were found in pumpkin plants and were not included in the analysis or figures.
1.3 Harvest data
Yields of ‘Baby Pam’ pumpkins were not significantly different by treatment. Reasons for unmarketable fruit included inadequate size, loss of stem during harvesting, scarring due to insect feeding, bacterial spots, and shriveled fruit due to dead vines. Most of the pumpkins harvested were marketable. There were no differences between marketable fruit due to treatment.
Yields of ‘Diplomat’ melons were not significantly different by treatment. Reasons for unmarketable fruit included splits and cracks, scarring due to insect feeding, raccoon/rodent bites, sunken spots and water-soaked lesions, rots and undeveloped fruit due to dead vines. Melons that were rotting in the fields were removed and counted as “field culls”. These were not weighed. There was a significant difference between marketable melons by treatment. Surprisingly, the highest percentage of marketable melons was from the unsprayed control plots (49%) and the lowest percentage recorded from the M3X plots.
